Soil high in calcium but End Blossom Rot
4 months ago
Featured Answer
Sort by:Oldest
Comments (30)
- 4 months ago
Related Professionals
Jackson Landscape Contractors · Brunswick Landscape Contractors · Overland Park Landscape Contractors · Point Pleasant Landscape Contractors · St. Louis Landscape Contractors · Braintree Landscape Contractors · View Park-Windsor Hills Landscape Contractors · Welby Landscape Contractors · Hueytown Landscape Contractors · Allentown Landscape Architects & Landscape Designers · Broomfield Landscape Contractors · Chesapeake Ranch Estates Landscape Contractors · Elkridge Landscape Contractors · Fort Myers Landscape Contractors · New Berlin Landscape Contractors- 4 months ago
- 4 months ago
- 4 months ago
- 4 months ago
- jkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 4 months agolast modified: 4 months agojkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- jkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 4 months agolast modified: 4 months agojkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 4 months agolast modified: 4 months agojkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 4 months ago
- 4 months agolast modified: 4 months agojkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- jkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 3 months ago
- jkhsdsu thanked daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)
- 2 months ago
Related Stories

COLORColor of the Week: Spring Blossom Yellow
Tired of winter yet? Bring on spring with our featured color of the week
Full Story
GARDENING GUIDESHow to Stop Worrying and Start Loving Clay Soil
Clay has many more benefits than you might imagine
Full Story
HOUSEPLANTSHigh-Impact Houseplants for First-Timers
These easygoing houseplants will forgive and forget if you skip a weekly watering
Full Story
GARDENING AND LANDSCAPING8 Rot-Resistant Woods for Your Outdoor Projects
No need for chemical treatments on your deck or pergola. These woods stand up to weather, insects and time beautifully on their own
Full Story
EDIBLE GARDENSHow to Grow Your Own Luscious Cherries
Nope, they’re not the easiest fruit to grow. But with spectacular blossoms and pies as possibilities, cherries are sure worth a try
Full Story
HOUSEPLANTSMother-in-Law's Tongue: Surprisingly Easy to Please
This low-maintenance, high-impact houseplant fits in with any design and can clear the air, too
Full Story
HOUSEPLANTSHow to Force Amaryllis Bulbs Indoors
Enjoy vibrant red blossoms even as gardens turn snowy white, by teaching this hardy repeat performer to ignore the calendar
Full Story
GARDENING GUIDESGreat Design Plant: Brittlebush Brightens Rocky, Dry Spots
Masses of cheerful golden flowers belie the tough nature of this highly drought-tolerant shrub
Full Story
GARDENING AND LANDSCAPINGHave a Ball With Hydrangeas
Even if you don't tinker with the hue by changing the soil, hydrangeas have an entertaining range of uses in all kinds of landscapes
Full Story
TILETop Tile Trends From the Coverings 2013 Show — the Wood Look
Get the beauty of wood while waving off potential splinters, rotting and long searches, thanks to eye-fooling ceramic and porcelain tiles
Full Story





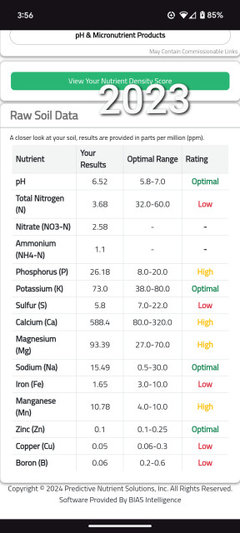
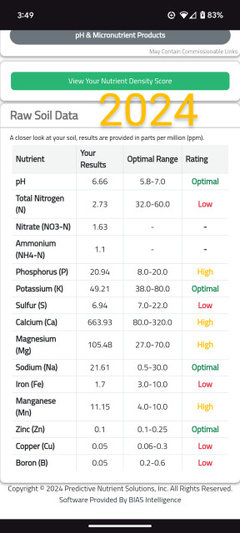





daninthedirt (USDA 9a, HZ9, CentTX, Sunset z30, Cfa)