Iowa Soil Test Results Help - Year 2
Wors
6 years ago
Featured Answer
Sort by:Oldest
Comments (14)
Wors
6 years agoRelated Professionals
West Milford Landscape Architects & Landscape Designers · Arlington Landscape Architects & Landscape Designers · Essex Landscape Architects & Landscape Designers · Hyattsville Landscape Architects & Landscape Designers · Jennings Landscape Architects & Landscape Designers · Newcastle Landscape Architects & Landscape Designers · Pottstown Landscape Contractors · Santa Maria Landscape Contractors · South Lake Tahoe Landscape Contractors · Teaneck Landscape Contractors · Chicago Ridge Landscape Contractors · Crowley Landscape Contractors · Vadnais Heights Landscape Contractors · Manassas Swimming Pool Builders · Thousand Oaks Swimming Pool Buildersbeckyinrichmond
6 years agodchall_san_antonio
6 years agobeckyinrichmond
6 years agoWors
6 years agobeckyinrichmond
6 years agoWors
6 years agobeckyinrichmond
6 years agobeckyinrichmond
6 years agodanielj_2009
6 years agoWors
6 years agobeckyinrichmond
6 years ago
Related Stories

DECLUTTERINGNew Year’s Resolutions for Minimalists
Pledge to do these 12 things in 2018 and you may find you’re more satisfied with less
Full Story
HOUZZ CALLTell Us Your New Year’s Resolutions for Your Home
Share your plans and dreams for your house this year — whether they involve organizing, remodeling or redecorating
Full Story
BATHROOM MAKEOVERSReader Bathroom: A New Shower and a Spa Look for $6,100 in Iowa
Over the course of 8 years and 2 renovations, a couple turn their dated bathroom into a relaxing and uplifting space
Full Story
GARDENING GUIDESHave Acidic Soil in Your Yard? Learn to Love Gardening Anyway
Look to acid-loving plants, like conifers and rhododendrons, to help your low-pH garden thrive
Full Story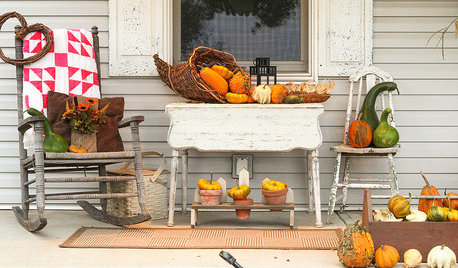
PORCHESA Peek at 2 Prettily Dressed Fall Porches
Pumpkins, fall flowers and flea market finds help two Ohio porches get into the seasonal spirit
Full Story
GARDENING GUIDES10 Solutions for Soggy Soil
If a too-wet garden is raining on your parade, try these water-loving plants and other ideas for handling all of that H2O
Full Story
GARDENING GUIDESGreat Design Plant: Try Blue Bells for Blooms in Dry Soil
This shrub’s violet-blue flowers and silvery foliage brighten low-water gardens all year long
Full Story
UNIVERSAL DESIGNAging-in-Place Resolutions for the New Year
How to make your home help you age gracefully right where you are
Full Story
GARDENING GUIDES8 Unthirsty Plants Help You Save Water in Style
Spend less effort and money on your landscape with drought-tolerant and native plants that liven up your yard
Full Story
DECLUTTERINGDownsizing Help: How to Edit Your Belongings
Learn what to take and what to toss if you're moving to a smaller home
Full Story








beckyinrichmond