Soil pH vs Water pH
absoluteblock
11 years ago
Related Stories

GARDENING GUIDESGrow a Beautiful Garden in Alkaline Soil
Got alkaline soil? Learn how to manage it and the many beautiful plants that will thrive in this ‘sweet’ soil
Full Story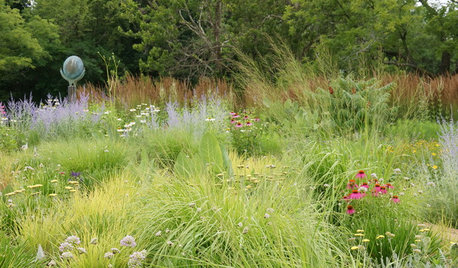
GARDENING GUIDESGet the Dirt on Your Garden’s Soil
Understand how your soil supports your plants so you can ensure your garden’s success
Full Story
GARDENING GUIDESGardening Solutions for Heavy Clay Soils
What’s a gardener to do with soil that’s easily compacted and has poor drainage? Find out here
Full Story
FARM YOUR YARDHow to Get Good Soil for Your Edible Garden
The nutrients in your soil feed the plants that feed you. Here are tips on getting it right — just in time for planting season
Full Story
GARDENING GUIDESHow to Stop Worrying and Start Loving Clay Soil
Clay has many more benefits than you might imagine
Full Story
CONTAINER GARDENSContainer Gardening Basics: The Dirt on Soil
Learn the types of potting soil available and the best mixes to help your containers thrive
Full Story
GARDENING GUIDESHave Acidic Soil in Your Yard? Learn to Love Gardening Anyway
Look to acid-loving plants, like conifers and rhododendrons, to help your low-pH garden thrive
Full Story
LANDSCAPE DESIGNSecrets of a Successful Water Garden
Relax. Having a water garden is much easier once you understand the basics
Full Story
GARDENING AND LANDSCAPINGHave a Ball With Hydrangeas
Even if you don't tinker with the hue by changing the soil, hydrangeas have an entertaining range of uses in all kinds of landscapes
Full Story
SPRING GARDENINGHow to Grow a Rose Garden in Pots
Everything can come up roses, even without a plot of soil in sight. This step-by-step guide to growing roses in containers shows you how
Full Story






User
absoluteblockOriginal Author
Related Professionals
Allen Landscape Architects & Landscape Designers · Elgin Landscape Contractors · Pottstown Landscape Contractors · Crystal Landscape Contractors · Dunwoody Landscape Contractors · Middleton Landscape Contractors · Seven Hills Landscape Contractors · South Lyon Landscape Contractors · Tewksbury Landscape Contractors · The Woodlands Landscape Contractors · Tinton Falls Landscape Contractors · Billerica Decks, Patios & Outdoor Enclosures · Brookfield Decks, Patios & Outdoor Enclosures · Hialeah Decks, Patios & Outdoor Enclosures · Saint Louis Park Decks, Patios & Outdoor EnclosuresKimmsr
ericwi
toxcrusadr
albert_135 39.17°N 119.76°W 4695ft.
tishtoshnm Zone 6/NM
absoluteblockOriginal Author
absoluteblockOriginal Author
TXEB
wayne_5 zone 6a Central Indiana
absoluteblockOriginal Author
TXEB
glib
TXEB
ericwi